Home
TGF-β, an important growth factor/cytokine involved in many human diseases and disorders
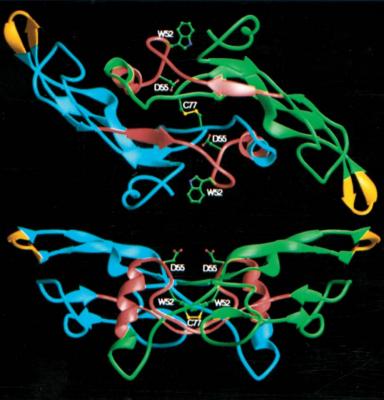
Transforming growth factor-β (TGF-β) is a family of structurally homologous dimeric proteins (three mammalian isoforms: TGF-β1, TGF-β2, and TGF-β3) (Fig. 1). They regulate multiple biological processes, including cell proliferation, extracellular matrix (ECM) synthesis, angiogenesis, immune response, apoptosis and differentiation. They have been implicated in the pathogenesis of chronic wounds, tissue fibrosis, cardiovascular disease, cancer, autoimmune diseases, and other disorders. The mission of Auxagen is to develop therapeutic agents based on its newly discovered technologies of TGF-β receptor antagonists and TGF-β enhancers.
Every year in the United States, >1.25 million people suffer from burns, 6.5 million have chronic skin ulcers caused by pressure, venous stasis or diabetes mellitus and 0.25 million have keloids sufficiently severe to require surgery. Burn treatment costs $1.8 billion per year in the US. The treatment of persons with chronic skin ulcers costs $13 billion per year in the US. The annual cost of diabetic peripheral neuropathy and/or neuropathic foot ulcers in the U.S. is $ 0.8 billion for type I diabetics and $10.1 billion for type II diabetics. Limb-sparing surgical procedures are also widely used. In spite of these large costs for the care and the treatment of diabetic foot ulcers, each year 82,000 limb amputations are still performed on US patients with diabetic ulcers because current therapy is not very effective. These surgical treatments cost about $ 0.3 billion per year in the US. Currently, there is no agent which has been shown to be effective for treating cutaneous wounds. As these costs suggest, there is an urgent need for developing effective agents to accelerate wound healing and reduce scarring or tissue fibrosis in patients with burn injuries, blast injuries, chronic skin ulcers, keloids and other similar disorders. New products to treat these patients will drive the market. Accumulating evidence indicates that transforming growth factor-β(TGF-β) (Fig. 1), a cytokine, provides an ideal target for developing novel therapeutic agents for many types of wounds including chronic wounds. It is produced at the wound site and is responsible for recruiting inflammatory cells and fibroblasts to the wound site, inhibiting epithelial cell growth (wound re-epithelialization) and stimulating extracellular matrix synthesis by fibroblasts (fibrosis) at the wound site.
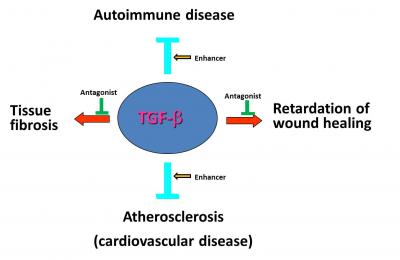
Our current understanding of the roles of TGF-β in impaired and normal wound healing, and tissue fibrosis (e.g., lung fibrosis, liver cirrhosis and fibrosis of other tissues) makes TGF-β an ideal target for developing novel therapeutic agents for these diseases in human (Fig. 2). However, until recently, no effective TGF-β antagonists were available for treating these diseases. Our newly developed synthetic TGF-β receptor antagonists potently and effectively block TGF-β binding to cells and tissues. They accelerate wound healing and reduce scarring when topically applied in a gel formulation to wounds in standard pig skin burn/excision and rabbit skin excision wound models. Our TGF-β receptor antagonist is more effective in enhancing wound healing in a standard pig skin burn wound model than platelet-derived growth factor-BB, the only FDA-approved wound healing agent. Our TGF-β receptor antagonists ameliorate and reverse lung fibrosis by inhalational administration in bleomycin- and radiation-induced lung fibrosis in mice. Auxagen’s new TGF-β receptor antagonists have excellent solubility, stability, potency and suitability for topical and parenteral administration using different formulations to treat wounds and tissue fibrosis (e.g., lung fibrosis) in humans, and be ready for FDA-approved preclinical and clinical trials.
Prevention and treatments of atherosclerosis and other diseases with Auxagen’s TGF-β enhancers